What is coal
Coal is a mineral whose extraction is necessary for human life. With its help, many processes are carried out that require thermal effects, and rare chemical elements are extracted. Previously, stoves were heated with it, but this is becoming more and more a thing of the past.
Externally, it is a shiny or matte, uneven black or dark brown specimen that resembles a stone, but is not one. Unlike stone, it crumbles in your hands when pressed and burns.
View this post on Instagram
Posted by Kyzyl Segodnya (@kyzylsegodnya) Oct 9, 2021 at 8:14 am PDT
Carbonation process
The term “carbonization” implies metamorphic transformations of carbon associated with an increase in the thickness of woody layers, tectonic movements and processes, as well as an increase in temperature depending on the depth of the strata.
An increase in pressure primarily changes the physical properties of coal, the chemical formula of which remains unchanged. In particular, its density, hardness, optical anisotropy and porosity change. An increase in temperature changes the very formula of coal towards an increase in carbon content and a decrease in oxygen and hydrogen. These chemical processes lead to an increase in the fuel properties of coal.
Classification of hard coals
The classification is based on the chemical and physical properties of the fossil. General division:
- Brown coal was formed later than other types. It has a low combustion temperature.
- Stone is the most common and used type. It is mined in open pits or in mines.
- Anthracite is the most ancient and hard representative. Has the highest combustion temperature of all types.
Brown coal differs from hard coal:
- color;
- lower nitrogen and carbon content;
- because it burns more easily;
- gives more smoke;
- produces less heat.
The mineral is divided according to the degree of carbonization and size. Based on these parameters, labeling was invented and implemented, reflecting the characteristics of a particular type of fossil. It is convenient for industrial use.
Watch an educational video review about the mineral:
By degree of enrichment
Before use, the mined rock is subjected to processing - enrichment. This is an increase in carbon content due to purification from mineral impurities, which increases flammability.
The wet method is often used - the fossil is loaded into an aqueous environment, in which separation into impurities and stone occurs. This is due to the fact that mineral supplements have a lower density. Machines for such enrichment are called jigging machines.
Industrial division according to the degree of mineral enrichment:
- Industrial products. Used in metallurgy.
- Concentrates. They produce energy for power plants and heating.
- Sludge is fine coal dust. It is used for the needs of the population; for this purpose it is pressed into briquettes.
According to the degree of carbonization
Coalification is the process of converting peat into brown coal or stone into anthracite. This is the degree of carbon saturation of a particular piece of fossil, which determines its properties - flammability, sinterability, heat of combustion. Depends on age - the younger it is, the lower the degree of carbonization.
Anthracite has the highest degree of carbonization, bright coals of grades M and D have the lowest, the remaining types are of medium degree.
By size
Mined minerals differ in length and width (this is called a fraction), therefore there is a classification where pieces of a certain size have their own name, abbreviated by one letter.
Sometimes this division is called a grade. Although a letter designation is used, this has nothing to do with brands.
Classification by size (fractions):
| Name | Size, mm |
| Slab (P) | More than 100 |
| Large (K) | 51–99 |
| Walnut (O) | 25–50 |
| Small (M) | 13–24 |
| Seed (C) | 6–12 |
| Shtyb (Sh) | Less than 6 |
| Private (R) | Unsorted, containing pieces of different sizes |
Coal grades
The mineral is divided into grades, the division is based on composition and combustion ability:
- long flame (D);
- gas (G);
- gas fatty (FG);
- fatty (F);
- coke fatty (QF);
- coke (K);
- lean sintering (OS);
- skinny (T);
- low-caking (SS);
- semi-anthracite (PA);
- anthracite (A).
Grade D hard coal is most often used in housing and communal services and the energy sector due to the following properties:
- a lot of volatile substances (from 39%);
- little sulfur (less than 0.5%);
- little ash;
- calorific value 4700–5400 kcal/kg is a good indicator;
- water content – 15–16%;
- high heat transfer.
The stone has a bright shine and is mined in the Krasnoyarsk Territory and Khakassia.
History of the formation of coal and its types
The entire process of coal formation can be divided into two main stages: the formation of peat and the actual process of coalification - the conversion of peat into coal.
Peat formed on vast water-covered areas from plant remains of varying degrees of decomposition. Some plants rotted completely to a gel-like state, while others retained their cellular structure. Their remains accumulated at the bottom of reservoirs, which gradually turned into swamps. A prerequisite for the formation of peat is the absence of oxygen. There was little oxygen under the water column; during the decomposition of the residues, hydrogen sulfide, methane and carbon dioxide were released, which contributed to the hardening of the residues. Peat formed.
History of coal. It all started many millions of years ago
But not all peatlands were converted to coal. The carbonization process requires: high pressure, high temperature and a long period of time. Depending on the presence of these conditions, the formation of coal occurred or not. First, the peat was carried over by sedimentary rocks, which increased the pressure and temperature inside the peat layer. Under such conditions, brown coal was formed - the first stage of coalification. In some areas, strata displacement occurred, causing brown coal seams to sink (some of the discovered deposits are at depths of more than 6,000 meters). In some places, these processes were accompanied by the rise of magma and volcanic eruptions. High pressure, lack of oxygen and high temperatures contributed to the fact that there was less and less moisture and natural gases in brown coal, and more and more carbon. As water and gases were displaced, brown coal turned into bituminous coal, then, in the presence of high temperatures, into anthracite. The main difference between brown coal and hard coal: brown coal contains more moisture and natural gases and less carbon, which affects the amount of heat released during combustion.
The age of coal is determined by the vegetation remains it contains. Sometimes the prints are very clear
Today, the age of coal deposits is determined by plant remains. The oldest ones date back to the Carboniferous period (345-280 million years ago). During this period, most of the coal basins of North America (eastern and central USA), central and western Europe, southern Africa, China, and India were formed. In Eurasia, most of the coal deposits were formed in the Permian period, some of the small coal basins in Europe date back to the Triassic period. The activity of coal formation increases towards the end of the Jurassic and in the Cretaceous. Around this time, deposits were formed in eastern Europe, the American Rocky Mountains, Indochina and central Asia. Later, mainly brown coals and peat deposits were formed.
Types of coal
Coal is classified according to its moisture, natural gas and carbon content. As the amount of carbon increases, its calorific value increases. The less moisture and volatile substances (gases), the better it tolerates storage and transportation.
Lignite is coal at the first stage of coalification. It differs from brown coal in the smaller amount of water (45%) in its composition and greater heat generation. The structure is fibrous, the color is from brown to black (higher quality). Most often used in the energy sector (at thermal power plants), it is rarely used for heating private houses, as it is poorly stored and has a low calorific value in conventional stoves.
Coal. Lignite. Has a loose layered structure
Subbitominous coal is black in color, has a less pronounced fibrous structure, has a higher calorific value compared to lignite, and has a lower moisture content (30%). It crumbles during transportation and dissipates in the open air. When burned, it emits 5-6 kW/kg. It is used both in the energy sector and in housing and communal services for heating.
Bituminous coal has the highest calorific value and does not lose its qualities during transportation and storage. When burning, it releases 7-9 kW/kg of heat. Some of its types are used for coking.
Anthracite is pitch-black coal. It has the highest hydrocarbon content. It is difficult to light, but it burns for a long time and without soot, and produces a large amount of heat (more than 9 kW/kg). It is anthracite that is most often used for heating.
Anthracite. It has a deep black color and a shiny surface.
Origin of coal
The mineral began to form long before the appearance of man. Approximate age: 400–200 million years. Until now, scientists do not have a clear opinion regarding which plant group formed the coal deposits. Most believe that they are fern-like.
There are 4 main theories trying to explain how coal was formed:
- The most common is the formation of peat, and then coal, due to the decay of ferns, mosses, and horsetails. However, this theory cannot explain fossil layers 400–700 meters thick. After all, to form 500 meters of fossils, 2000 meters of peat are required, i.e. plants of the same species had to grow on the territory for millions of years without changes in weather conditions.
- Thermal theory - slow smoldering of plant residues in an environment with a low oxygen content with gradual transformation into ordinary coal, and then into stone. However, plant parts would not be preserved inside the fossils.
- Sea water version. Having fallen into the ocean, the plants underwent a long process of carbonization, being under pressure and without oxygen. The theory is confirmed by marine finds - algae, sand.
- Abiogenic - coal appeared by heating methane in the presence of hydrogen and carbon dioxide. According to this theory, the finds in the layers are not the remains of plants, but pyrolytic graphite, therefore it is impossible to reliably determine the age of the mined minerals.
When mining and processing mineral deposits, surprising discoveries are sometimes encountered:
- vertical tree trunks;
- huge stone blocks weighing up to 73 kg, of metamorphic or volcanic origin;
- products made of gold and metal, which indicates the ongoing process of coal formation;
- mollusks, shells, annelids;
- round objects resembling dinosaur eggs.
Because of its origin, coal is called canned solar energy - plants are able to accumulate it in leaves and shoots.
Petrographic composition of coal
Coal occurs in layers with a layered structure. Individual layers consist of solid organic rock of different structure and origin. It is customary to distinguish between macro- and microcomponents of formations. They differ from each other not only in composition, but also in appearance and microscopic structure.
Macrocomponents of coal
These components of coal occur in layers, lenses, or prisms within the fossiliferous rock. They were formed from various plant species during the process of peat metamorphism. Most often, changes took place under anaerobic conditions (without access to oxygen).
Macronutrients do not have a specific chemical structure. At one time, cellulose, lignin and other plant tissues underwent a process of gelification - transformation into a jelly-like substance. Then it hardened and became like stone. Under a microscope, in some cases you can see fossilized spores, cell walls, and plant fibers.
Fossilized plants or their imprints in coal can be detected without a microscope. This is not uncommon. Some mines even have their own museums of such artifacts, and coal with fossils is being sold on the Internet. For example, in 1998, an entire forest was discovered in a coal seam in Illinois, America, preserving its original structure. Its area reaches 10 km2, and its age is 307 million years. Huge ferns, horsetails, and remains of reptiles and arthropods have been identified in this forest.
Main macroelements of coal:
- Vitren A shiny black material, brittle, fissured, with a conchoidal fracture, of a dense, homogeneous structure. Vitrene is formed from lignin and cellulose under decomposition conditions with limited oxygen access. It goes through a gelification process. In young coals, a cellular structure is detected under a microscope, while in more mature coals, vitrain is a homogeneous mass. The component has good sintering properties and increases the coking properties of coal.
- Klaren The shine of the material is weaker than that of Vitrain. Claren consists of a translucent gelified mass with a heterogeneous structure. It is soft, with single cracks. The ash content in it is 1.2% with a slight predominance of aluminum oxide (Al2O3). Clarine is formed from the cuticle and spores. It lies in thick layers and is classified as a sintering material. The component acts as glue, holding together different parts of the coal rock.
- Duren This is a hard black coal with a matte sheen. Its structure is dense, homogeneous, the texture and fracture are granular. The composition of duren includes yellow formed elements - pollen, spores, resin bodies. The remains of the plant body have a black tint. You can examine the elements under a microscope or magnifying glass. Duren has a high ash content, does not sinter, and is difficult to enrich.
- Fusin The structure of the rock is fibrous, loose, reminiscent of charcoal. Under a magnifying glass or microscope, cells and plant fibers, sometimes annual rings, are clearly visible. The inner part of the fibers is filled with minerals - calcite or pyrite. Fusin is formed from wood residues that have decomposed in the presence of oxygen. In layers it occurs in the form of lenses or prisms. The material does not sinter, has a high ash content, and a low release of volatile substances during combustion.
The ratio of macroelements in coal affects its quality and methods of use. For fuel and coke production, rock containing vitrain and clarine is better suited. Duren and fusin are more often used to produce resinous substances, tar, and gas generation.
Microcomponents of coal
Coal microcomponents, or macerals, are tiny organic particles that can only be seen under a microscope. Like macrocomponents, they do not have a specific chemical structure. The composition includes cyclic aromatic carbons in different proportions. The classification is based on the genesis of substances from plant residues, their hardness, gloss, light reflection and other physical properties.
The quantity and ratio of microcomponents of coal determine its grade and the characteristics of the metamorphism of the layers. This affects how the fossil is used and its characteristics.
There are several groups of macerals:
- Showcases
- Semivitrinitis
- Liptinites
- Inertinites
Each group includes several more varieties of microcomponents. We will tell you more about them later.
Showcases
It is a group of chemicals formed from lignin and cellulose. They are hard, with a smooth shiny surface, and contain aromatic compounds with a cyclic structure. The color ranges from black and dark gray to almost transparent, depending on the degree of metamorphism.
Vitrinites lost a significant part of hydrogen and oxygen during their genesis; carbon predominates in their composition. When heated, they melt and release medium to low amounts of volatile substances.
The group includes:
- Telinite The material consists of the walls of woody cells, which are clearly visualized under a microscope. There is a lot of it in bituminized coal; in mature fossils the amount decreases.
- Collinite The main cementing substance of Vitren.
- Vitrodentrinite Formed from fragments of telinite and collinite with a diameter of about 10 microns.
Vitrinites are one of the most common and important organic constituents of coal. The color and relief of these macerals are used as a standard for identifying other groups. They are the least ash-rich, as well as fragile and dense (1300-1400 kg/m3). Coal with a high content of vitrinites is a valuable fuel and material for coke production.
Semivitrinitis
This group of microcomponents is formed from cellulose and lignin, with an admixture of wood residues (fusain). The surface of semivitrinites is smooth, the color is gray (always lighter than that of vitrinites). When heated, substances soften, but do not become plastic.
The group of semivitrinites includes:
- Semitelinitis
- Semicollinitis
According to physical characteristics, semivitrinite occupies an intermediate position between vitrinite and inertinite. Its presence indicates low or medium metamorphism of coal. Such a fossil usually contains less carbon and more oxygen and hydrogen. With a high content of substances, the heat of combustion decreases and the ability to oxidize increases. But usually in coal the amount of semivitrinites does not exceed 1-3%, which does not affect the quality of the material.
Liptinites
The group of liptinites, or exinites, was formed from plant lipids. The color depends on the origin and degree of carbonization; it can be dark brown, black and gray. The structure of liptinites practically does not change during the transformation of peat into brown and hard coals. They are not amenable to humification and gelification. Therefore, under a microscope, plant particles are clearly visible - spores, pollen, cuticle, wax.
The group includes 6 organic substances:
- Sporinitis The structure is dominated by plant spores. This is a durable material that binds the elements of duren together.
- Cutinite Forms from fossilized plant cuticle. It is durable and contains a large amount of hydrogen. When burned, many volatile substances are released.
- Rubberite It is formed from tree resin and wax, scattered throughout the rock or occurs in layers. Rubberite contains a lot of hydrogen. It can dissolve in alcohol, benzene. Resin and bitumen can be obtained from it.
- Suberinite This is a yellow colored component formed from cortical tissue. It occurs in the form of crusts enveloping the main rock layer.
- Alginite Alginite comes from lower plants, algae, protozoa and bacteria rich in lipids. It is part of only a special type of coal - sapropelites. They formed at the bottom of fresh and salt water bodies. The substance is very hard, rich in hydrogen, and black in color.
- Liptodetrinite Formed from small destroyed particles (detritus) of plants. It is a mixture of all the components described above.
The density of liptinites is relatively low, 1200-1300 kg/m3. When burned, they release many volatile substances. This group of macerals produces high-quality coke.
Inertinites
They are formed from plant debris (usually wood) that decomposed in the presence of oxygen. Inertinites occur in thick layers on the sites of old dried swamps. They have a matte shine, cellulose fibers are visible in the structure, and the wood pattern is preserved. The color of the substances is light, from yellow to white.
The carbon content in inertinites is high, and the hydrogen content is low. When burned, they release very few volatile substances and do not sinter. They contain a large amount of aromatic carbohydrates. The density of this type of macerals is high, 1400-1500 kg/m3.
The inertinite group includes 6 substances:
- Fusinite It is characterized by a preserved cellular structure and cellular structure. The internal cavities of cells can be filled with organic and mineral substances. Fusinite ranks first in carbon content among all coal components.
- Micronite It was formed from resinous trees, found in large quantities in the coal of the Paleozoic era, long-flame varieties. Micronite is scattered in layers in the form of microscopic grains and can fill voids between the walls of plant cells. Over time, it turns into a substance little different from vitrinite.
- Macrinite is rare in coal. It is an amorphous mass that glues other components together.
- Sclerotinitis It is formed from the remains of fungi. Sclerotinitis has the shape of oval bodies with clear outlines and a porous structure. Inclusion sizes range from 10 microns to 80 microns. Sclerotinite occurs in coal of the Permian period.
- Semifusinite It consists of wood remains with a partially preserved cellular structure and, in its characteristics, occupies an intermediate position between vitrinites and inertinites.
- Inertodetrinite This is a mixture of fragments of all macerals of the inertinite group with sizes up to 20 microns.
Microcomponents make up the bulk of coal. During the process of metamorphism, they gradually decompose, lose their structure and turn into pure crystalline carbon. Other elements pass into the mineral part of the coal seam. We'll talk about it further.
Deposits and rock mining
The fossil is widespread - its reserves account for 15% of all land. Three leading countries in coal mining:
- The USA is a world leader. The percentage of deposits is 23 – that’s more than 1,600 billion tons.
- Russia. Deposits are estimated at 13%.
- China. The figure is approaching 11%.
In Russia, hard coal is mined in the Kemerovo region, in the Kuznetsk deposit, where 640 billion tons of minerals are located. The discovery took place in 1721 by M. Volkov. In 1842, P. Chikhachev assessed the reserves of the basin. In the 2nd half of the 19th century, coal began to be mined in Kuzbass.
In Yakutia there is the Elga deposit, its reserves amount to about 2 billion tons, in Tyva the Elegot deposits are promising for development. Other basins are Lensky, Tungussky, Irkutsk, South Yakutsky, Pechersky.
In the USA, the largest deposits are located in the state of Illinois (reserves of more than 360 million tons).
Kazakhstan contains 162 billion tons of fossil fuels. One of the largest fields is Ekibastuz, other basins:
- Shubarkol and Kyzyltal - 2 billion tons each;
- Shoptykol, Mamyt and Eginsay – 1 billion tons each;
- Karazhyr – 890 million tons.
View this post on Instagram
Publication from COAL FIREWOOD TRANSPORTATION (@vezet19_124) January 29, 2020 at 7:26 PST
Deposits of hard coal are indicated on the map in the form of a certain symbol - a black square, and brown coal - a shaded one.
In the Republic of Khakassia, the main deposit is located in the Minusinsk Basin. Development has been going on since 1904. Khakassian coal is mined in the Izykh and Chernogorsk basins.
In Africa, the mineral is mined in the following areas:
- Zimbabwe;
- Mozambique;
- SOUTH AFRICA.
Coal exporting countries by rating, volume indicated in millions of tons per year:
- Australia - 193;
- China – 91;
- South Africa – 69.3;
- Indonesia – 66.4;
- USA – 44.1;
- Russia – 41;
- Colombia - 37.1;
- Canada – 30.6;
- Kazakhstan – 28;
- Poland – 23.
Watch a program about stone mining in Russia:
Physico-chemical properties of stone
The main element of stone is carbon, which is why it burns well and for a long time. In addition, it contains water. The ratio of elements depends on the age of the mineral:
| Coal name | % water | % carbon | % volatile matter | How does it ignite? | How it burns | Heat dissipation |
| Brown | Up to 43 | Up to 45 | Up to 50 | Fine | Fine | Low |
| Stone | Up to 12 | 75–95 | Up to 32 | Great | Fine | Average |
| Anthracite | 1–3 | 85–95 | Less than 9 | With difficulties | Weak, no smoke due to low volume of volatile impurities | High |
Properties:
- specific gravity – 1.5–1.7 g/cm2;
- combustion temperature in the furnace – 700–1100 °C;
- shine – pronounced, with a metallic tint, less often – golden;
- specific heat capacity – 1300 J/(kg × K);
- specific heat of combustion – 5700 kcal/kg;
- fracture – uneven, conchoidal;
- hardness of coal ash on the Mohs scale – 2;
- The shelf life of coal is 6–18 months.
Watch a program where a scientist talks in an entertaining way about the properties of the mineral:
Simple chemical elements
Almost all chemical elements in coal are in bound form. They are part of organic and inorganic compounds.
Of greatest practical importance are:
- Carbon (C): 75-92% Carbon is the main element of organic compounds. The heat of combustion of coal depends on its quantity. It is part of the organic part of the material. The content of the element increases during the process of metamorphism. The most carbon is in anthracite (up to 97%), less in brown coal (60-70%).
- Hydrogen (H): 2.5-5.7% The heating value of hydrogen is 4 times higher than that of carbon. But in its pure form this element becomes explosive. The amount of substance decreases depending on the degree of metamorphism. For brown and hard coal it is higher than for anthracite. There is a lot of hydrogen in sapropelites - types of coal formed from lower types of plants.
- Oxygen (O): 1.5-15% The amount of oxygen decreases during metamorphism. In peat this element is about 40%, in brown coal 10-30%, in anthracite – 1-2%. With a high oxygen content, the processes of oxidation and combustion of the material are accelerated.
- Nitrogen (N): 1-3% The element is of organic origin. Its percentage decreases during the genesis of coal.
- Sulfur (S): 0-4% Sulfur can enter coal both during the decomposition of plant residues and from the surrounding rock layers. When fuel burns, it oxidizes and turns into sulfur dioxide SO2. When the gas dissolves in water, sulfuric acid is formed. It damages the walls of boilers. Therefore, the amount of sulfur in fuel coal is strictly regulated. The most harmful sulfur compound is sulfide (S2O). About 70-80% of salt turns into a gaseous state when heated. Sulfur dioxide and hydrogen sulfide are released, polluting the atmosphere.
- Phosphorus (P): up to 0.03% Phosphorus is one of the elements that is part of organic substances. Its content must be regulated in coke. If phosphorus gets into the steel, the quality of the alloy decreases sharply.
- Chlorine (Cl): 0.015-0.15% The chlorine content in coals ranges from 0.015 to 0.15%. In so-called “salty coals” the figure can reach 1%. If the rate is above 0.3%, fuel combustion becomes difficult. When oxidized and dissolved in water, chlorine forms hydrochloric acid. It causes metal corrosion and damage to boiler walls.
- Arsenic (As) Arsenic enters coal from groundwater, and only a small portion is of organic origin. This element is found in high concentrations in “spots” in some deposits. When fuel is burned, it can be released into ash and air. At high levels, arsenic harms the environment and provokes cancer.
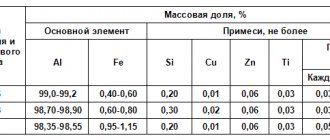
GOST 32464-2013 regulates the content of a number of elements in coal:
- Sulfur – up to 2.8% (enriched), 3% (unenriched), 4.6% (ordinary)
- Chlorine – up to 0.6%
- Arsenic – up to 0.02%
Application of hard coal in industry
For hard coal used in industry, GOST 32464-2013 is applied - a decree that describes technical requirements, methods for determining the chemical composition, and classification according to various parameters.
The use of the fossil is based on the pyrolysis reaction of coal - heating without any reagents. In modern industry, various chemical additives are increasingly used to speed up the reaction. Pyrolysis stages:
- condensation;
- polymerization;
- aromatization;
- alkylation.
Separately distinguished:
- Low-temperature pyrolysis occurring at 500–600 °C. This is semi-coking.
- High temperature process, or coking. It runs at 900–1100 °C.
All coking products are divided into 3 groups:
- Solid - coke. Used in ferrous and non-ferrous metallurgy.
- Liquid – coal tar. More than 250 chemical compounds are obtained from it. The main ones are technical oils, synthetic fuels, naphthalene, benzene, ammonia. Dyes, TNT and saccharin are made from toluene.
- Gaseous – pyrolysis gas. Alternative source of energy and heat.
When processing fossils, the following products used in industry can be obtained:
- vanadium, sulfur, zinc, lead;
- xylene and benzene - used in the paint and varnish industry;
- solid fuel used to heat houses and ensure the operation of enterprises;
- liquid fuel - obtained by liquefying solid fuel;
- illuminating gas used for lighting;
- ash - used in construction.
The fossil belongs to the 4th hazard class, as a flammable substance capable of heating.
For personal use, fossils are used to generate heat. Where there is little wood or it is difficult to prepare firewood, you can heat the bathhouse with coal. Many houses still have stoves. Premises heated with coal warm up well and are acceptable even for harsh winters.
Lighting coal is not easy - you need an armful of firewood, wood chips or splinters, straw, paper. First, several firewood are laid, chips (splinters) are placed on them, and straw is placed on top. They set fire to the paper and use it to set the straw on fire. Then the wood gradually lights up. The rest of the firewood is added as space becomes available. When it's hot, they put in coal. The preparation process pays off with a long combustion time and good heat transfer.
Coal ash is not used as a fertilizer - it contains few nutrients and may contain heavy metal impurities. The only acceptable solution is to use it on highly alkaline soils to normalize acidity - ash acidifies the soil.
Some people suggest grilling shish kebab on it, but you shouldn't do that.
We invite you to watch a foreign program about the situation with mineral resources in Europe:
How to make at home
Very often, the production of charcoal at home is carried out by people who own metal forging workshops. Homemade biofuel is made for domestic needs: cooking on the grill, refueling the forge. Before you make charcoal with your own hands, you need to choose a manufacturing method and organize production workshops, taking into account fire safety rules. You can make charcoal at home using available materials. At the same time, the manufacturing technology of the material is often not followed. In the production of this product, pits, barrels and stoves are used. Before you set up a coal production workshop yourself, you need to evaluate the amount of costs and profitability of the business project.
In the hole
This method assumes the presence of a pit located at a far distance from buildings. Its depth should be at least 150 cm, width - 80 cm. To make charcoal in the pit, you need to light a fire from small branches. It needs to be placed in a hole. Medium-sized pieces are thrown into the fire. After burning the wood, the pit must be covered with a covering and left for several days to cool. The resulting products can be removed within 2 days.
In a barrel
When making charcoal in a barrel, it is necessary to use containers made of heat-resistant materials. The bottom of the metal barrel is reinforced with bricks. Between them there is a fire, on which wooden blanks are placed. A metal grate is placed over the pile of firewood to allow heat and flames to pass through. This design allows you to produce several portions of coal in a barrel.
In the oven
You can make coal in a standard stove. To do this, you need to place the wood in the fuel compartment and heat it to 550 °C. You must wait until the wood turns red. The resulting fuel is removed using tongs, placed in a metal container and covered with a lid. After cooling, the product can be packaged in bags and used at home.