Induction heating principle
The operation of an induction furnace is based on the transformer principle of energy exchange. The inductor is made of a copper tube, which is then twisted into a multi-turn coil. An alternating current is supplied to the primary circuit of the inductor, which leads to the formation of an alternating magnetic field around it. Under the influence of a magnetic field, an electric field arises in a body located inside the inductor, which subsequently leads to a heating process. The power, and therefore the heat generated by an induction crucible melting furnace, directly depends on the frequency of the alternating magnetic field. Therefore, for efficient operation, the furnace requires high frequency currents.
Application of induction furnaces
Induction heating can be used to work with any material: metal, slag, gas, etc. The main advantage of its use is non-contact heat transfer. Also, induction heating allows you to achieve almost any heating speed - it all depends on the power of the generator powering the furnace. Heat losses with such heating are minimal. The maximum temperature to which an object can be heated in an oven is limited only by the resistance of the refractory material. The process of non-contact heat transfer to the heated material makes it possible to produce heating in a vacuum environment.
According to reviews from metallurgists, the scope of application of induction furnaces is somewhat limited due to existing shortcomings. The disadvantages of a crucible furnace include:
- high price for electrical equipment;
- cold slags, complicating the refining process;
- reduced stability of the lining during temperature changes between heats.
A little about melting
In a deep vacuum, the high-purity metal being melted can be heated exactly to the melting temperature or slightly higher, and kept at it for some time so that tiny, literally a few atoms, remains of crystallites melt. Then the metal can be allowed to cool slightly below its melting point - it will remain liquid, like a supersaturated solution without a seed crystal. If we now pour the metal, also in a vacuum, into a mold made of a chemically absolutely inert material, in which a seed crystal of the same metal is placed, then, observing all the subtleties of this technology, we will obtain a single-crystal casting with unique properties.
In amateur conditions, vacuum melting, alas, is not feasible. In order to properly make a crucible for melting metal yourself, you need to take into account a number of features of melting in a non-inert chemical gas environment. The melted metal, firstly, interacts with air, causing part of it to be lost to the formation of oxide, which is especially important when melting scrap precious metals: at its melting temperature (1060 degrees Celsius), even gold noticeably oxidizes. To compensate to some extent for oxidation, the crucible must provide a reducing environment for the melt or be chemically inert if the metal is melted with a clean open flame, see below.
Secondly, so that the metal in the crucible does not freeze until it is brought to the casting mold, so that the remnants of the original crystallites do not spoil the casting, and the melt acquires sufficient fluidity, the metal in the crucible is overheated. For example, the melting point of zinc is 440 degrees, and its foundry temperature is 600. Aluminum, respectively, 660 and 800. Since overheating of the metal after melting takes some time, degassing of the melt also occurs at the same time, this is the third thing.
Recovery
In metallurgy, atomic carbon C, carbon monoxide CO (carbon monoxide) and hydrogen H are used as reducing agents. The latter is most often an accidental guest, because for this purpose it is too active and is absorbed by metals without forming chemical compounds with them in large quantities, which spoils the casting material. For example, solid platinum at room temperature can absorb up to 800 volumes of hydrogen. A platinum blank in a hydrogen atmosphere literally swells before our eyes, cracks and falls into pieces. If you take them out of the hydrogen chamber and heat them, hydrogen will be released back.
Note: in a similar way, but in smaller quantities, metals absorb/emit other gases, e.g. nitrogen. This is why degassing of the melt is required, see also below.
A noticeable proportion of hydrogen reduction occurs when heated by an open flame of a gas burner, upon its contact with a less heated surface. The metal does not deteriorate - the absorbed hydrogen is released and burned later in the smelting process. But, if the crucible material is also prone to gas absorption, it may crack and burst during melting; this must be kept in mind.
CO reduction is noticeable if the metal in the crucible is melted by the open flame of a liquid (gasoline, kerosene, diesel) burner, for the same reasons. Liquid fuel burns much slower than gas, and its afterburning zone extends several cm from the burner nozzle. Reduction with carbon monoxide is the cleanest from the point of view of the metal: it does not spoil the metal and does not produce by-products with a strong excess of the reducing agent. Therefore, CO reduction is widely used in metallurgy when smelting metal from ore, but no one has yet figured out how to make a crucible furnace (see below), in which oxidation compensation would be completely provided by CO.
Atomic carbon is a reducing agent energetic enough to compensate for oxidation. It is also not difficult to create a reducing environment in a crucible using C: it is enough to introduce free carbon in one or another allotropic modification into the composition of its material or make the entire crucible from a heat-resistant and mechanically sufficiently strong allotrope C; graphite is one of them. When reducing C, there is a danger of carburization of the melt, but graphite releases very little atomic carbon when heated. If you heat the metal in a graphite crucible with a gas flame, then the excess C will immediately find a more “tasty” H for it and the danger of carburization will be reduced to zero. And for other heating methods (see below), you can select the dimensions, configuration of the crucible and the addition of graphite to its material so that there is simply no excess C under any conceivable melting mode. This is a very valuable property of graphite, keep it in mind too.
Note: the coefficient of thermal expansion of TKR graphite is negative, which significantly compensates for the thermal expansion of the crucible, increases its durability and increases its service life. Also valuable quality.
Excerpt
So, it’s clear why the melt in the crucible needs to be overheated and held. Although metal casting is a completely different topic, it still needs to be mentioned here that the melt holding time should be observed quite accurately. Chemically pure metals are almost never used in practice, for example. gold 9999 wears out very quickly; The exception is electrical copper and zinc for galvanizing, the cleaner they are, the better. Most often they use the so-called. eutectic alloys; eg steel is a eutectic of iron and carbon, and duralumin is a complex eutectic of several components. If the melt is allowed to sit, the structure of the eutectic in the casting will change and the finished product will be spoiled. The holding time is especially critical for bronze and brass: they need to be cast immediately, as soon as the play of the melt in the crucible apparently changes and becomes calmer. Remember how the engineer Telegin in A. N. Tolstoy’s “Walking Through Torment” was worried that the bronze would not wear out?
Read also: Adapter for LED strip 220V
In relation to the manufacture of a homemade crucible, degassing of the melt during exposure is significant in that at this time it (the crucible) experiences significant dynamic loads from bubbles of released gases and/or the play of the melt itself. That is, make the crucible withstand a large amount of thermal deformation and, if recovery is required, a small amount. Its material must also be viscous enough to withstand shock waves from bursting bubbles and shocks from melt jets. It is this circumstance that explains the low durability and reliability of homemade graphite crucibles (see below).
Scheme of a crucible induction furnace
The induction crucible furnace has the following structure.
The main element of the furnace is the crucible (7), covered with a lid (1). The crucible is located inside a heating inductor (3), made in the form of a multi-turn coil. The coil is a copper tube, inside of which water is constantly circulating for cooling purposes. The magnetic flux from the inductor passes through magnetic cores (4), which are made of special transformer steel. The rotating unit (2) is provided for tilting the furnace during pouring of the molten liquid. The furnace is installed on a mello structure (5). Cooling is carried out using water cooling hoses (6). An auxiliary platform (8) is used to service the furnace.
The crucible furnace circuit also includes a transformer, capacitors, a control unit and a gas pumping system. The crucible electric furnace is powered by currents with a frequency of 50 Hz.
General characteristics of induction crucible furnaces.[edit]
Induction heating
- heating of bodies in an electromagnetic field due to the thermal effect of an electric current flowing through the heated body and excited in it due to the phenomenon of electromagnetic induction. In this case, the current in the heated product is called induced or induced current. Induced installations are called electrothermal devices designed for induction heating of bodies or melting of certain materials. An induction furnace is a part of an induction installation that includes an inductor, a frame, a heating or melting chamber, a vacuum system, mechanisms for tilting the furnace or moving heated products in space, etc. An induction crucible furnace (IFR), which is otherwise called a coreless induction furnace, is a melting crucible, usually cylindrical in shape, made of refractory material and placed in the cavity of an inductor connected to an alternating current source. The metal charge is loaded into a crucible and, absorbing electromagnetic energy, melts.
Advantages
crucible melting furnaces: 1. Energy release directly into the charge, without intermediate heating elements;
2. Intense electrodynamic circulation of the melt in the crucible, ensuring rapid melting of small batches and waste, equalization of temperature throughout the volume of the bath and the absence of local overheating, guaranteeing the production of multicomponent alloys that are homogeneous in chemical composition; 3. The fundamental possibility of creating any atmosphere (oxidizing, reducing or neutral) in the furnace at any pressure; 4. High performance achieved due to high power densities, especially at medium frequencies; 5. The possibility of completely draining the metal from the crucible and the relatively small mass of the furnace lining, which creates conditions for reducing the thermal inertia of the furnace due to a decrease in the heat accumulated by the lining. Furnaces of this type are convenient for periodic operation with breaks between melts and provide the ability to quickly switch from one grade of alloy to another; 6. Simplicity and ease of maintenance of the furnace, control and adjustment of the smelting process, ample opportunities for mechanization and automation of the process; 7. High hygiene of the melting process and low air pollution. The disadvantages
of crucible furnaces include the relatively low temperature of the slag brought onto the melt surface for the purpose of its technological processing. The slag in the ITP is heated by the metal, so its temperature is always lower, as well as the relatively low durability of the lining at high melt temperatures and the presence of thermal shifts (sharp fluctuations in the temperature of the lining when the metal is completely drained). However, the advantages of ITP over other melting units are significant, and they are widely used in a wide variety of industries. Depending on whether the smelting process takes place in air or in a protective atmosphere, furnaces are distinguished:
- open (melting in air),
- vacuum (melting in a vacuum),
- compressor (melting under excess pressure).
By organizing the process over time:
- periodic action
- semi-continuous
- continuous action
According to the design of the melting crucible:
- with ceramic (lined) crucible,
- with a conductive metal crucible,
- with conductive graphite crucible,
- with a cold (water-cooled) metal crucible.
Features of internal structural elements
Most often, the inductor is made from a round tube. But there are situations in which round copper tubing is not applicable. In certain cases, profiled elements are used to design an induction crucible furnace, due to which the magnetic dissipation flux is reduced. The inductor tubes are insulated among themselves with fiberglass impregnated with a special varnish. The protected turns are compressed by blocks made of dielectric material. The inductor and crucible, placed inside the coil, are installed on a pallet made of refractory bricks or heat-resistant concrete. In industrial conditions, the crucible manufacturing process takes place directly in the furnace. In this case, the assembled inductor is installed on a pallet and insulated with asbestos. After this, the pallet is filled with refractory powder, which is compacted using a pneumatic installation. The gap between the template installed on the bottom and the inductor is filled with powders made of refractory materials.
The lining of the area above the inductor is provided by refractory bricks. The collar and drainage chute are also lined with heat-resistant bricks. The operation of an induction crucible furnace occurs under the most difficult conditions, so increased demands are placed on the quality of the heat-resistant materials used. The durability of the lining is affected by the composition of the refractory mass, the operating mode and the applied frequency of the electric current. As a rule, the crucible can withstand up to 100 heats and then fails.
Lining of an induction crucible furnace.[edit]
The lining of a crucible furnace consists of a melting crucible with a drain sock, the so-called “collar”, a hearth, a lid and a layer of thermal insulation. The melting crucible is one of the most critical components of the furnace, which largely determines its operational reliability. Therefore, the following requirements apply to the crucible and the lining materials used:
- refractory materials must have high heat resistance and fire resistance, as well as chemical resistance to molten metal and slag at operating temperatures;
- the crucible material must maintain insulating properties (that is, have minimal electrical conductivity) over the entire temperature range (1600..1700°C) for ferrous metals);
- the crucible must have a minimum wall thickness to obtain a high electrical efficiency;
- the crucible must be mechanically strong under conditions of high temperatures, high metallostatic effects, significant mechanical forces arising from tilting the furnace, shock loads occurring during loading and deposition of the charge and cleaning the crucible;
- the crucible material must have a low coefficient of linear (volume) expansion to prevent the occurrence of cracks in the crucible under conditions of a high temperature gradient in the wall (up to 3·104 °C/m) and to reduce thermal stresses in the crucible;
- the design and manufacturing technology of the lining and thermal insulation of the furnace must provide conditions for the implementation of an unsintered (buffer) outer layer during the entire furnace campaign, adjacent to the inductor, and excluding the formation of through cracks and penetration of the melt to the turns of the inductor.
Currently, the following methods are used in the production of ITP: 1. Stuffing according to a template directly in the furnace, when a template welded from sheet steel in the shape of the inner surface of the crucible is installed on the hearth exactly on the axis of the furnace, powdered refractory masses are poured into the gap between the inductor and the template, and compacted layer by layer with a pneumatic or electric tamper. 2. Manufacturing the lining using the out-of-furnace method: the crucibles are pressed, compacted or molded in special collapsible molds, then the crucibles are installed in the furnace inductor and the side space is filled with powdered refractory material, which prevents the breakthrough of liquid metal to the inductor through through cracks that can form in pre-fired crucibles . With this method, changing the lining can be done faster, which reduces furnace downtime. 3. Making linings from shaped refractory products. The thickness of the products (rings, blocks, sectional tongue-and-groove products, standard wedge-shaped bricks) should be such that during laying a space (annular gap) of 25 - 30 mm in size does not form between the outer wall of the masonry and the turns of the inductor to create a buffer layer of powdered materials. 4. Layer welding of the lining by shotcrete or plasma spraying of contact working layers onto a lining manufactured by any method. The spraying method makes it possible to produce a chemically clean and highly refractory contact surface of the lining, in accordance with the requirements of the melted alloys. For ITP, acidic, basic and neutral linings are used, the composition of which is very diverse. This allows for a given melting process to select the appropriate lining materials, the formulation of refractory masses and manufacturing technology in accordance with the previously listed requirements. Acid lining is made from siliceous refractory materials (quartz sand, quartzite, ground silica brick) with a silicon oxide content of at least 93 - 98%. Sulfite-cellulose extract is used as a binding (strengthening) material, and a 1 - 1.5% solution of boric acid is added as a mineralizer. Grain composition of the refractory mass: 5% grains 3 - 2 mm, 50% grains 2 - 0.5 mm, 45% grains <0.5 mm. The acidic lining can withstand 80 – 100 heats. The main lining is made of magnesite refractories in a pre-sintered or fused state, that is, having the greatest consistency of volume. To reduce shrinkage at high temperatures (1500–1600 °C) and provide some growth at medium temperatures (1150–1400 °C), which prevents the formation of shrinkage cracks, mineralizers such as storage ore, quartz sand or quartzite are used. Clay is used as a binder (up to 3% by weight of magnesite) moistened with an aqueous solution of liquid glass or molasses (up to 12%). The best refractory mass in terms of grain composition is considered to be: 50% grains 6 - 0.5 mm, 15% grains 0.5 - 0.18 mm, 35% grains <0.18 mm. Data on the service life of the main lining are extremely contradictory and vary for crucibles of different capacities. It should be noted that the durability of the main lining is lower than that of the acidic lining, and there is also a drawback: the formation of cracks. Neutral lining is characterized by a high content of amphoteric oxides (Al2O3, ZnO2, Cr2O3). In many cases, it has higher refractory characteristics than acidic or basic, and makes it possible to smelt heat-resistant alloys and refractory metals in ITP. Currently, neutral linings are made from magnesite-chromite refractories, electrocorundum, zirconium dioxide and zircon (zirconium orthosilicate ZrSiO4). It is also possible to manufacture crucibles of a neutral composition from some refractory compounds (nitrides, carbides, silicides, borides, sulfides), which may be promising for melting small quantities of chemically pure refractory metals in a vacuum and in reducing or neutral environments. Melting in large-capacity crucibles, which would justify the use of such expensive lining materials, is not yet used.
The furnace cover, which serves to reduce heat losses by radiation, is made of structural steel and lined from the inside. The lid is opened manually or using a lever system (on small stoves), or using a special drive (hydro- or electromechanical).
The hearth of the furnace, which serves as the base on which the crucible is installed, is usually made of fireclay bricks or blocks (for large furnaces) or of asbestos-cement slabs laid one on top of the other (for small furnaces of small capacity).
Design of external elements
The frame of the melting crucible furnace is the base to which all its elements are attached. On large industrial devices, the frame takes the form of a continuous casing. All frame parts must have high strength, due to the influence of the electromagnetic field of the inductor on them. The shell, under certain conditions, can heat up in the same way as the material in the oven. To reduce heating, it is rational to make the frame from non-conductive materials. However, since dielectric materials are expensive, the frame material is usually steel. The steel structure is divided into several elements, which, in turn, are isolated from each other. To reduce the electromagnetic field near the frame, screens are used. A protective screen is installed between the inductor and the furnace body. The screen has the shape of a cylinder and is made of aluminum or copper.
The rotary unit is an important design element. The main requirement for the turning mechanism is to provide a tilt for complete drainage of the metal. Different turning mechanisms can be used. Small ovens use a manual or electric winch. Industrial furnaces are tilted using a crane beam. Large-volume ovens can be equipped with a hydraulic tilt drive.
The lid that covers the crucible furnace for smelting serves to maintain the temperature inside the unit at a higher level. However, given that the furnace can be covered only after complete melting of the charge, the use of a cover is not mandatory.
Frame of an induction crucible furnace.[edit]
The frame (casing) of the furnace serves as a structural basis for fastening all the main elements of the furnace. At the same time, two main requirements are imposed on it: ensuring maximum rigidity of the entire furnace structure as a whole and minimal power absorption by the frame elements, since they are located in the magnetic stray field of the inductor. Currently, the following basic frame schemes are used in crucible furnaces: 1. A frame in the shape of a rectangular parallelepiped, the ribs of which are made of non-magnetic material (for example, from a duralumin corner or non-magnetic steel), and the edges are covered with an asbestos cement sheet. Small-capacity furnaces (less than 0.5 tons) and laboratory furnaces are made with such frames. In order to reduce the heating of the metal corners of the frame, its individual metal elements are isolated from each other with insulating gaskets to eliminate ring currents in the frame frame. The inductor in such a frame is usually attached to the lower and upper asbestos-cement slabs. 2. The metal frame is usually cylindrical in shape, made in the form of a continuous winding of thick steel sheet with cutouts (“windows”) for access to the inductor or in the form of a “squirrel cage” formed by vertical metal posts welded to the upper and lower support posts. Between the posts there is access to the inductor. Such frames are mainly used in medium- and large-capacity ovens.
Making a stove with your own hands
Induction furnaces are widely used not only in industry, but also in everyday life. You can find circuit diagrams for a large number of homemade devices, but some of them, at best, simply will not work, and at worst, they will harm the health of their creator. Many amateurs warn about such consequences. In everyday life, the induction heating method is used in the following devices:
- channel furnace for metal melting;
- crucible induction furnace is the simplest to design, and therefore the most popular among enthusiasts, judging by the reviews;
- water heating boiler, the operation of which is based on the induction method;
- induction hobs that compete with popular gas stoves.
Channel furnace
This type of furnace is used to produce high-quality cast iron, as well as for melting duralumin and non-ferrous special alloys. A channel furnace with a power of up to 3 kW is manufactured independently from a welding transformer, the frequency of which corresponds to industrial frequency. Such a furnace allows you to melt a piece of bronze or copper weighing up to half a kilogram. The channel furnace also allows you to melt duralumin, but you must take into account that the melting must be followed by an “aging” process. This process can take up to 2 weeks and depends on the composition of the alloy.
To manufacture the furnace, the primary winding of the welding transformer is left unchanged, and a ring-type crucible is placed in place of the secondary winding. The best material for the crucible of a small channel furnace is electroporcelain. Other options are not suitable due to low strength and dielectric losses. According to reviews from amateur metallurgists, the problem is that it is not possible to process electroporcelain on your own, and it is very unlikely to find a suitable element on sale. It is precisely because of the scarce crucible that the channel furnace has not found wide use among enthusiasts, although this type of furnace has an efficiency of more than 90%.
Crucible induction furnace
A self-made crucible furnace is used primarily for the purification of valuable metals. For example, if you have a radio connector made in the Soviet Union, you can extract a certain amount of gold from its contacts. Using external heating, this result cannot be achieved.
In addition to gold mining, such a furnace is often used for the purpose of uniform heating of metal, which is required for high-quality hardening. By changing the position of the part in the inductor and adjusting its power, you can achieve a given temperature in a specific area of the metal. It is important that the use of such a furnace will be quite inexpensive, because almost all the energy is directed to the process of heating the part.
Heating methods
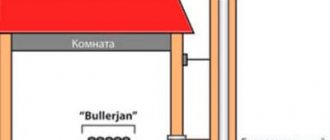
If you need to melt more than 150-200 g of metal at a time, then you will need to build a crucible furnace next to the crucible, otherwise it will be very difficult to achieve homogeneity of the melt and high quality casting. The exception is low-melting and easily recoverable lead: up to 20-30 kg of it can be melted at a time at home. A relative exception is zinc for hot galvanizing; its melt in a crucible without a furnace can be up to 2-2.5 kg, but borax must be sprinkled on top of it so that the surface of the melt is completely covered with its fluidized layer. Steel fasteners are thrown into the melt through a layer of borax.
The optimal method in all respects for heating the crucible in a furnace is with gas, pos. 1 in Fig., but a gas crucible furnace is a rather complex structure, although it can easily be made independently. The most suitable crucible for a gas furnace is a graphite ceramic crucible, because its material has fairly high thermal conductivity. If there are particularly high requirements for metal purity, it is better to use a neutral ceramic crucible. When lower for fusible metals - cast iron, as it conducts heat better and thereby saves fuel. Graphite crucibles are placed in a gas furnace only if strong reduction of old oxidized metal is required, and the danger of carburization is insignificant, for example, when melting silver extracted from the earth for refining
Methods for melting metal in a crucible
For low-melting metals, the electric crucible furnace, pos. 2; it may be the so-called ohmic (with heating by a nichrome spiral) or induction, with heating from an electromagnetic oscillation generator, see below. Only ceramic neutral or, to a limited extent, graphite crucibles are suitable for induction furnaces.
If the crucible contains more than 2-2.5 kg of metal, then according to safety rules the crucible furnace must be made tiltable (item 3), because and 1 kg of melt spilled on the floor is already a big disaster. On the contrary, it is preferable to heat metal in small jewelry crucibles without a furnace, directly with the flame of a burner, pos. 4. In this case, the crucible is held throughout the melting process with a special spring grip, pos. 5 and 6.
Note: silver and its alloys, as well as lead on sinkers, can be melted at home in quantities of up to 15-20 g, using instead of a crucible... a food-grade stainless steel spoon, see fig. on right. For safety, then it is necessary to make gaskets for the jaws of the vice with longitudinal cuts under the handle of the spoon. The flame is exclusively gas; gasoline can burn a spoon.
Read also: How to sharpen ceramic knives
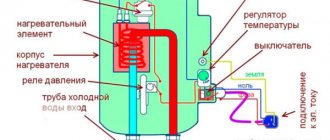
Electric heating
Ohmic crucible furnaces are mainly used for smelting lead or tin. For more refractory metals, they turn out to be uneconomical, but up to 20 kg of lead can be melted at a time in a home crucible electric furnace; how to make your own electric crucible for melting lead, see for example. video:
Video: electric crucible for melting lead
Melting aluminum in a crucible turns out to be more profitable by induction due to its high electrical conductivity, but this trick no longer works with copper - its temperature and latent heat of fusion are much higher. With the induction melting method, the metal is heated by Foucault eddy currents, for which the crucible with it is placed in an EMF coil of thick copper wire, powered by alternating current from an electromagnetic oscillation generator. How to make a generator with your own hands for inductively heating small amounts of metal, for example, for trinkets, is described in other materials, or, for example, see next. video guide.
Video: DIY induction heating
Induction crucible furnace for aluminum melting
With an increase in the amount of melted metal, not only does the required power of the generator increase, but its optimal frequency also decreases, this affects the so-called. surface effect (skin effect) in metal. If 100-200 g of aluminum can be melted into EMF from any homemade generator for inductive heating, then installation of 1.5-2 kg of duralumin or magnesium alloy is already a solid structure, see fig. on right. If you intend to work with aluminum, then think carefully - is it worth building something like this? Wouldn't it be easier to use a mini gas furnace for melting small quantities of aluminum alloys, see for example. video clip
Video: mini furnace for melting aluminum
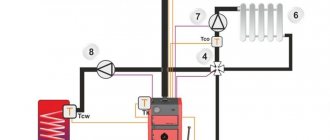
Induction boilers
Induction water heating boilers have every chance of replacing conventional boilers in the future. Users consider the high price to be a disadvantage of such a water heater, but at the same time, systematizing numerous reviews, several advantages can be identified:
- Reliability. The boiler does not have an electric spiral, which is the weak link of a conventional boiler.
- The efficiency is almost 100%.
- Safety. Electrical access to the boiler body is impossible due to the design features.
- The device does not require special grounding.
- Resistant to power surges.
- Does not form scale.
- Durability. The boiler can operate without maintenance for about 30 years.
Homemade water heating boiler
The basis of such a water heater is a power transformer with a power of up to 1.5 kW, the primary winding of which is designed for a voltage of 220 V. A transformer from a tube color TV is ideal. The secondary winding should be removed, and the number of turns of the primary should be increased.
Craftsmen advise and warn: using such a homemade device is unsafe, so the transformer should be grounded, and the device itself should be connected through a high-speed RCD.