Wood pyrolysis: concept and products
Wood pyrolysis (dry distillation of wood) is the decomposition of wood when it is heated to a temperature of 450 °C without oxygen. As a result of this process, gaseous and liquid (including tree resin) products are formed, as well as a solid residue - charcoal.
The technology and process of wood pyrolysis is one of the first technological chemical processes known to mankind. Starting from the mid-12th century, this technology was widely used in our country to produce pine resin (which was used to impregnate ropes and tar wooden ships). This trade was then called tar smoking.
When metallurgy began to develop, another trade arose, which was also based on the dry pyrolysis of wood - charcoaling. In this case, the final product of pyrolysis was charcoal.
The beginning of the spread of industrial use of wood pyrolysis can be considered the 19th century.
The main product of pyrolysis then was acetic acid, and only hardwood was used as a raw material.
The process of wood pyrolysis is based on various free radical reactions of thermal destruction of cellulose, lignin and hemicelluloses, occurring at temperatures from 200 to 400°C. Wood pyrolysis is an exothermic process, which produces a fairly large amount of heat (about 1150 kJ/kg).
The technological scheme of wood pyrolysis includes the following stages:
- cutting wood raw materials into pieces
- drying cut wood
- direct pyrolysis
- cooling and stabilization of coal (to prevent spontaneous combustion);
- complete condensation of vapors of volatile products.
The longest and most energy-intensive stage of all those listed above is drying the wood to a moisture content of 15%.
Wood pyrolysis products
Currently, hardwood is usually used to carry out the process of wood pyrolysis, but sometimes (mainly during complex processing of raw materials) coniferous wood is also used. Modern pyrolysis technologies make it possible to obtain from birch wood:
- charcoal - 24-25% charcoal,
- liquid waste (so-called slurry) - 50-55%
- gaseous products - 22-23%
The larger the size of the pieces of wood taken for pyrolysis, the larger the solid residue will be. The charcoal obtained as a result of pyrolysis, after the sorting procedure according to the size of the pieces, is sent directly to the consumer or for processing.
When processing the liquid obtained as a result of pyrolysis, wood resin (which is approximately 7-10%) settles and at the same time numerous transformations of the components occur.
A wide range of valuable products can be distinguished from resin. As a rule, acetic acid is isolated from the liquid.
It is usually extracted from the liquid by extraction, and then, through rectification and thorough chemical purification, it is processed into a food product ready for sale.
Gaseous products of wood pyrolysis (non-condensable gases) include:
- carbon dioxide CO2 (approximately 45-55%)
- carbon monoxide CO (28-32%)
- hydrogen H2 (1-2%)
- methane CH4 (8-21%)
- other hydrocarbons (1.5-3.0%).
The composition of gaseous products of wood pyrolysis depends on the pyrolysis temperature, speed and heating method. The heat of combustion of gaseous products ranges from 3.05 to 15.2 MJ/m³. All of the above factors, as well as the type of wood, its quality and humidity, determine the final yield of pyrolysis products.
With increasing temperature, the yield of wood resin and gaseous products increases, but the yield of charcoal, alcohol products and acetic acid decreases. Coal, as a result of increasing temperature, is formed with a higher percentage of carbon. The average yield of the main products of wood pyrolysis is (based on dry wood):
- charcoal - 23-24%
- tree resin - 10-14%
- acetic acid - 5-7%
The technology for wood pyrolysis is quite diverse, but most of the devices used in world practice are hopelessly outdated and do not meet all modern requirements.
In addition, the need for wood pyrolysis is constantly decreasing, since destroying such environmentally friendly raw materials is quite wasteful.
However, the technology of sawdust pyrolysis is beginning to become increasingly popular.
Pyrolysis of sawdust
Pyrolysis of sawdust is the most profitable way to dispose of wood waste. Thanks to this technology, waste from the wood processing industry does not need to be taken to a landfill for disposal, but can be used to generate heat and electricity.
In recent years, this use of wood waste has begun to be seen as an excellent alternative to traditional fuels. All this is directly related to the fact that sawdust as a fuel has a number of advantages:
- they belong to renewable sources of thermal energy
- are completely CO2 neutral
- There is practically no sulfur in sawdust
- it is possible to burn wet waste (containing up to 55 - 60% moisture)
- The corrosiveness of flue gases is quite low
- low, compared to fossil fuels, price of raw materials
Using wood waste as fuel is not only much less harmful to the environment, but also saves money.
This way of saving non-renewable natural resources can allow Russia to get closer to more developed countries in terms of such an indicator as the specific energy intensity of industrial production, which makes it extremely attractive.
And all this leads to the fact that sawdust pyrolysis technologies have been constantly developing and improving in recent years.
Sawdust disposal
The service life of most devices used for dry distillation of wood in industry is ending, and there is a need to update the technical equipment at enterprises. The processing of sawdust is becoming an increasingly popular pyrolysis technology. Companies install special equipment for this.
Constantly disposing of trees is a wasteful activity, but recycling sawdust is a rational use of a resource.
The product obtained from pyrolysis is a cheap fuel that can be produced regularly if there is enough wood.
Wood pyrolysis: process description, raw materials, gas composition
Most of the wood is made up of organic compounds.
Under the influence of high temperatures under vacuum or minimal air flow, they break down into solid, semi-liquid and gaseous components.
This process is called dry distillation, tar smoking, pyrolysis. It differs from combustion in the greater preservation of decomposed components. Chemical reactions take place very quickly and without the formation of flames or smoke.
Distillation technology appeared in the 12th century . At that time, pine and other coniferous species were used as raw materials. From them they extracted resin for impregnating wooden parts of ships and ropes, and charcoal. In the 19th century, acetic acid was produced through the destruction of carbon-containing mass.
The essence of the process
Normal combustion of wood with the participation of oxygen leads to ignition and evaporation of the released gases, the complete destruction of solid components, turning them into smoke, soot, ash and ash. The flame temperature reaches 1000°C. Pyrolysis is also thermal destruction.
The result of this process is the formation of decomposition products of lignin, cellulose and hemicellulose. Dry distillation is carried out in a closed space at a constant temperature of 250–450°C; the resulting gases and liquid components released are immediately removed and cooled. The process is accompanied by a large release of heat, but smoke and soot are not formed .
The resulting residues can then be used for industrial or household purposes.
Procurement of raw materials
The starting material used is wood that is of little use for other needs, and production waste, including sawdust. It is customary to distinguish several groups of raw materials:
- hardwoods: beech, birch, elm, oak, hornbeam, maple, ash;
- soft-leaved: linden, alder, aspen, poplar;
- conifers: larch, pine, cedar, fir, spruce.
There are strict production regulations, according to which all timber arriving at processing plants is sawed, cut and collected into piles of a certain width and height in special warehouses. They are placed on flat areas, providing access to air and lighting.
Preparation for pyrolysis involves preliminary drying . This process can be difficult, especially when working with solid aspen or poplar, which, with increased dampness, are prone to fungus infection and the development of rotting.
Drying is carried out naturally in the ventilated area of warehouses. To speed up the process, the material is split into small pieces. Air-dry wood is considered suitable for further processing: about 12–15%.
Sometimes quick drying is used: the massif is crushed, placed in an oven or blown with dry hot air.
Equipment
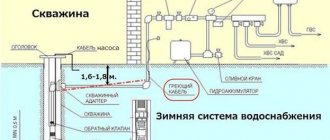
Wood decomposition is carried out in retort ovens. The bodies are cylindrical containers welded from metal. The thickness of their walls is about 15 mm. There is a loading opening, inside there are gratings for placing raw materials, a system for supplying coolants, removing and cooling the released liquid products, gases and charcoal, at the bottom there is a port for unloading the resulting components.
Industrial equipment comes in different sizes. More often, large furnaces are used, the combustion chambers of which are about 2–2.5 m in diameter. The following heating systems are used:
- external: the metal walls of the retort heat up, starting the process of wood decomposition;
- internal heat supply: the temperature is maintained by a mixture of released gases; their efficiency is usually several times lower than that of those heated from the outside.
Equipment can be continuous, semi-continuous and batch . In the first case, all stages of the cycle occur simultaneously.
When the next batch of wood comes inside, the finished coal is unloaded from the outlet. Semi-continuous devices have an orderly process. First, the first batch of raw materials undergoes complete processing; after unloading, the next one arrives.
The type of equipment affects the rate at which wood decomposes.
There are boilers similar to industrial ones, but more compact. They are designed for pyrolysis on a small scale.
The result of distillation is influenced by the conditions in which the process occurs and the state of the incoming material.
Distillation process
If the loaded batch of wood is not dry enough, after grinding it is dried in a closed chamber at a temperature of 130°C. This stage is the most energy-intensive, since it requires an external heat source. The evaporation of moisture is accompanied by the primary decomposition of some wood components.
Further heating to 155–200°C leads to the beginning of the release and evaporation of gaseous substances.
Direct decomposition of the entire mass of material occurs upon subsequent heating to 280–420°C. In this case, resins, acetic acid, and carbonyl compounds are released and removed. At the same time, charcoal is formed.
The final stage is calcination. The temperature inside the retort rises to 500°C and above. Heavy resins and carbon compounds are removed from wood residue. Then the products are cooled and unloaded from the chamber.
The amount of material obtained, the speed of the process and energy costs depend on the type and size of pieces of wood and equipment systems . Fast pyrolysis using external heat sources produces larger quantities of coal and high purity at relatively low energy costs.
Pyrolysis products
The main components for which wood is distilled are coal and acetic acid.
Coal
The amount of solid residue obtained depends on the type of wood. For hardwood beech and birch, the yield is about 25% of the primary material. In coniferous species it is slightly higher. Soft-leaved plants produce the least coal. Pyrolysis of sawdust produces coal flour. At the same time, the yield of liquid residue is higher.
High-quality coal has no cracks, brown or whitish deposits, and burns without smoke. A defective product is obtained when the pyrolysis technology is violated: insufficient temperature, air penetration into the furnace.
Charcoal is an environmentally friendly and affordable type of fuel, which is used for industrial and household stoves and home fireplaces.
It releases a large amount of heat, produces virtually no by-products or odor during combustion, and has a low cost.
Coal is used in the metallurgical industry, agriculture, for the production of filters, plastics, dyes, glass, and medicines.
Condensate
Liquid pyrolysis products, or condensate, contain resinous compounds called slurry. Its amount reaches 50% of the total residue and depends on the type and moisture content of the wood, and the type of pyrolysis.
The liquid contains ketones, resins, aldehydes, alcohols, esters, and water.
As a result of multi-stage reactions, acetic acid is formed from it - a compound used in the chemical, textile, pharmaceutical, cosmetology, and food industries.
Formic and butyric acids, acetone, methyl and isopropyl alcohols, formaldehyde, and resins are obtained from the liquid.
Gases
Gaseous compounds released as a result of distillation are formed in an amount of 20–25%. The composition of pyrolysis gases includes:
- CO: 40–50%;
- CO2: 28–38%;
- CH4: 8–20%;
- H2: 1–2%;
- carbon impurities: about 1%.
On average, during dry distillation of 1 m³ of wood, about 70–80 m³ of gaseous compounds are released.
Distilling wood at home
You can also perform pyrolysis of wood or its waste at home. In this case, you will only be able to get coal.
Large-volume metal barrels are used as a retort . Chemical containers must not be taken. A clean container with several small holes to allow gases to escape is required.
First, prepare the platform:
- A large sheet of iron is placed on the ground.
- Several refractory bricks are placed on the edge, and firewood is laid between them.
- They make a fire.
The barrel is filled with dried chopped wood and hermetically sealed. After that, it is placed on a platform with a fire. When the container gets hot and oxidation begins, gas will come out of the holes. The process may take several hours.
When the gas output stops, the barrel is left on the fire for 30 minutes . After cooling, remove the lid and take out the finished charcoal. It can be used to kindle baths, home stoves, and fireplaces. After distilling the sawdust, the resulting flour is added to garden soil and used to process plant cuts.
Pyrolysis of wood at home
When performing dry distillation of forest resources at home, the final product can only be charcoal. But the process does not require special tools.
You need a large, clean metal barrel (to serve as a retort) with 2-3 holes on the sides or on the lid through which gases are released. The barrel is filled with wood and hermetically sealed.
An iron sheet is placed on the ground. Dry brushwood is placed on it, 2 fireproof bricks are placed along the edge and a fire is made. Next, secure the barrel over the fire and wait for the coal to be cooked. The gas escaping from the holes is harmful and is not advisable to be near.
Dry distillation of wood at home is not difficult. This is not a labor-intensive process, most of which consists of waiting for the result.
Wood pyrolysis
Wood pyrolysis is also called dry distillation. This process is the decomposition of wood under high temperature conditions of up to 450 °C without oxygen. As a result of this process, gaseous and liquid (including tree resin) products are obtained, as well as solid material - charcoal.
Wood pyrolysis technology
Pyrolysis is one of the first technological chemical processes known to mankind. Back in the middle of the 12th century, this technology was actively used to obtain pine resin, which was used to impregnate ropes and tar wooden ships. This process was then called tar smoking.
With the beginning of the development of the metallurgical industry, another industry arose, based on the dry pyrolysis of timber - charcoaling. In this process, the final material was charcoal.
The beginning of the spread of industrial use of wood pyrolysis can be considered the 19th century. The main product of pyrolysis in those days was acetic acid.
The raw materials were only deciduous timber.
The pyrolysis process is based on various free radical reactions of thermal destruction of cellulose, lignin and hemicelluloses. These reactions occur at temperatures ranging from 200 to 400°C. Wood pyrolysis is an exothermic process that produces a large amount of heat (approximately 1150 kJ/kg).
The technological scheme for pyrolysis of timber consists of the following stages:
- wood shredding
- drying chopped wood
- pyrolysis
- cooling and stabilizing coal to prevent spontaneous combustion
- the process of condensation of vapors of volatile products.
The most time-consuming and energy-consuming stage is drying the wood to a moisture level of 15%. Drying is carried out at temperatures of 130-155°C using external heat. This removes water from the timber and changes some of the wood components.
After this, the wood begins to decompose. This happens within the temperature range from 155 to 280°C. At this stage, its least stable components disintegrate. In this case, carbon dioxide, carbon monoxide, and acetic acid are released.
Then the temperature rises to 280-455°C. Under these conditions, evaporation begins and the formation of the main volume of wood decomposition products begins.
In this case, there is an active release of heat (exothermic process) with the release of large amounts of CO2, CO, CH4, ethers, carbonyl compounds, hydrocarbons, acetic acid, its homologues and methanol. At the very end, the resin is removed.
Then the calcination of the wood residue begins. The temperature rises to more than 500°C. During this process, heavy tar is released and removed, as well as CO2, H2, CO and hydrocarbons. This is the end of pyrolysis, and the resulting residue is charcoal.
The volume of wood pyrolysis products obtained varies greatly, it all depends on the size of the pieces of timber, the temperature of the process, its duration, and the moisture level of the raw materials.
Devices for pyrolysis
This process takes place in retorts. A retort is an all-welded cylindrical metal vessel. Inside, it has a diameter of 2.5 to 2.9 m, and the wall thickness is 15 mm.
At the top of the device there is a loading device for raw materials, and at the bottom there is a conical part and an unloading device for coal. The retort has a height of about 25 m. The retort is equipped with four pipes.
The vapor-gas mixture is removed through the upper pipe, the coolant is introduced through the second, the third removes heated gases from the coal cooling area, and through the fourth, the lowest, cold gases are introduced that cool the coal.
Retorts are:
- continuous action
- periodic action
- semi-continuous action.
In addition, based on the heating principle, there are:
- devices with internal heating. In such devices, heat is supplied to the timber from the coolant through direct contact. The coolant is hot flue gases, which are forced into the device. In this case, the pyrolysis process is carried out more gently, but the volume of decomposition products is approximately 7-10 times less
- devices with external heating. In such devices, heat is supplied through the metal walls of retorts, which are heated by hot flue gases.
The most common are semi-continuous devices. Wood is loaded into them periodically, in small quantities at equal intervals of time. The steam-gas mixture is taken continuously, and coal is unloaded periodically, in portions.
In continuous devices, all stages of the process occur simultaneously: drying occurs in the upper part, then the timber is heated to the decomposition temperature, in the middle part the wood decomposes, and in the lower part the coal is calcined and cooled.
Rapid pyrolysis of wood
A fairly common type of pyrolysis can be called fast pyrolysis, during which thermal energy is supplied to the original mixture at high speed. The entire process takes place without oxygen.
The process of slow pyrolysis can be compared to bringing water to the boiling point, but the fast pyrolysis method can be compared to dropping a drop of water into hot oil, which is otherwise called explosive boiling.
The main features of rapid pyrolysis of timber:
- the possibility of forming a closed, continuously flowing technological production process
- significant “purity” of the final pyrolysis products, which is achieved due to the absence of a resinization stage
- low energy intensity of this process compared to other types of pyrolysis
- in this process, a large amount of thermal energy is released (exothermic reactions during rapid pyrolysis are superior to endothermic ones).
Yield of thermal decomposition products
Raw materialsThermal decomposition products, wt. % by weight a. With. e.coal,tars,volatile components,gases,water
| Spruce | wood | 37,9 | 15,3 | 6,3 | 18,2 | 22,3 |
| bark | 42,5 | 18,4 | 1,9 | 19,8 | 17,4 | |
| Pine | wood | 38,0 | 16,7 | 6,2 | 17,7 | 21,4 |
| bark | 40,5 | 18,2 | 5,7 | 19,7 | 15,9 | |
| Birch | wood | 33,6 | 14,3 | 12,3 | 17,0 | 22,8 |
| bark | 37,9 | 24,0 | 4,7 | 18,6 | 14,8 | |
| Aspen | wood | 33,0 | 16,0 | 7,3 | 20,4 | 23,3 |
We use sawdust pyrolysis at home to produce fuel
Any wood waste can be used to produce flammable pyrolysis gas.
In terms of volume/heat release ratio, pyrolysis gas is inferior to natural gas (the difference is 25–50% in favor of natural gas), so it can be used in conventional boilers , but in larger volumes.
In addition, pyrolysis gas can be used as fuel for automobile engines, but the power will be lower by:
- 20–40% compared to liquefied natural gas (propane, methane, butane);
- 30–50% compared to gasoline.
Nevertheless, the car will drive , and fuel costs will be significantly lower. After all, when driving in normal modes, the engine is never used at full power.
The only disadvantage of using pyrolysis gas will be lower acceleration during acceleration, that is, it will be more difficult to overtake cars on the highway.
In this article we will talk about:
- what is pyrolysis;
- what equipment is used for pyrolysis;
- how pyrolysis gases are purified;
- How are pyrolysis gases used?
What is pyrolysis?
Pyrolysis is the thermal decomposition of wood , as a result of which cellulose breaks down into:
- hydrogen;
- carbon monoxide (CO);
- nitrogen;
- water vapor;
- carbon dioxide (CO2).
Pyrolysis begins at a temperature of 300–400 degrees and occurs in the absence of oxygen.
However, a self-sustaining reaction requires a small amount of oxygen to keep some of the wood burning and maintaining a high temperature. Therefore, in pyrolysis plants the process occurs with a strong oxygen deficiency (15–30% of the required).
If more oxygen is supplied, the pyrolysis gases will burn directly in the installation.
During the pyrolysis process,
wood breaks down into various gases and a small amount of inorganic residues, so the formation of ash in pyrolysis plants is tens of times less than during conventional combustion of wood waste.
The efficiency of pyrolysis directly depends on the moisture content of the wood - the wetter the wood, the more heat is needed for thermal decomposition and the more water vapor in the pyrolysis gas.
Therefore, wood waste is pre-dried in special installations, which you can read about in the article Equipment for wood processing.
The process of pyrolysis can occur in any organic matter, but most often wood waste, branches and other similar materials are used to produce gas.
The calorific value of the finished fuel is affected by the density and humidity of the source material, and by density we mean the specific gravity of the wood.
The wetter the fuel, the more energy will be expended to maintain the pyrolysis process and the higher the water vapor content at the outlet.
Along with sawdust, shavings and chips from healthy or diseased wood, as well as any waste from dry wood processing and processing, can be loaded into the gas generator.
In addition, even fallen leaves and tree bark can be used as fuel, but their calorific value is much lower than that of healthy wood, so the operating time of the gas generator on one load of fuel will be much shorter.
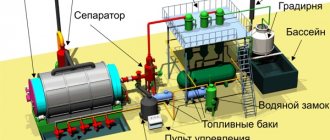
Gas generating units
Apparatuses and devices for producing pyrolysis gas are called gas generator units.
They are a sealed stove with adjustable air supply and the ability to shut off the chimney.
To reduce the requirements for the chimney, air is forced into them using centrifugal pumps.
Moreover, they either use a pump with variable performance (this is done using a frequency converter), or install several pumps to ensure maximum air supply in ignition mode.
When the contents of the installation flare up,
the air supply is reduced, leaving only the minimum necessary to maintain the optimal temperature.
As a result, thick black smoke begins to emerge from the installation, which contains unburned carbon (soot) and pyrolysis gases.
This gas cannot be used immediately due to the large amount of soot, so it is purified using various devices, the most popular of which are cyclones.
The soot collected by the cyclone can either be loaded with wood waste into a gas generating plant or sold to tire manufacturers. After all, soot is one of the main components, the share of which reaches 30%.
In addition, water vapor is removed from the pyrolysis gas, which increases its combustion temperature. To do this, the gas is passed through a cooler, where the water vapor condenses into water droplets.
As water accumulates, it is drained through a special tap located at the bottom of the cooler.
After this, the gas is fed into a fine filter, which uses electrostatic devices, cardboard cartridges and a container of water.
Electrostatic devices operate due to the different electrical capacitance of gas and any solid particles.
Under the influence of static electricity, solid particles stick to the positive or negative electrode (depending on the electrical potential of the particle), and the gas passes through without obstacles.
The electrodes must be periodically
cleaned of soot adhering to them.
Cardboard filters work on the principle of a mesh - they allow gases and solid particles to pass through them, which are smaller than the size of the pores that permeate the entire cartridge, so it has to be changed regularly, which is not cheap.
The water in the container does not retain the gas, but does trap the smallest solid particles of soot. As the water becomes dirty, drain it and add new water. The drained water is evaporated to produce soot, which is then either sent to a gasification plant or sold to tire manufacturers.
Despite the fact that sawdust is also wood and the basic principles of obtaining gas from it are the same, conventional gas generators cannot be used .
This is due to the peculiarities of air movement through the fuel mass.
Large wood waste does not adhere tightly to each other , so air between them easily passes in any direction.
When the fuel container is filled with sawdust, air passes between them very little, just as pyrolysis gas cannot pass through.
Therefore, in installations designed to produce pyrolysis gas from sawdust and shavings, air is supplied in several places , and the gas outlet hole is located at the top.
When planning to buy a gas generator, do not forget to check whether it is designed to work on sawdust and shavings. After all, generators designed for processing large waste do not work well with small ones , but those designed for small waste can process large ones.
Cost of gasifiers
We have prepared a table that includes the most popular models of gas generator units.
Most of them are designed for use with heating boilers, but they can be adapted for other purposes.
In addition, we included a semi-industrial gas generator for a car in the list. To clarify its parameters and select the most suitable one for your car, you need to contact the supplier using the link given in the table.
| Model | Additional functions | Power/Performance | Price thousand rubles | Seller or manufacturer website |
| KDO-1 | A gas generator with a combustion chamber and a heat exchanger for heating water (boiler). Possible purchase without heat exchanger and combustion chamber | 15-100 kW | 169 | bmpa.info |
| KSDO-125 | Gas generator with control panel and water heating boiler. If desired, another boiler can be installed, and it is also possible to purchase a complex without a boiler | 125 kW | 495 | tayur-kotly.ru |
| KH-100V | Gasifier without additional equipment | 100 m3 of gas per hour | 2700 | ooo-smog.promportal.su |
| UGK | Gas generator for car | 50-100 kW | 460 | belgorod.promportal.su |
| UDSO-60 | Gasifier without additional equipment | 60 kW | 300 | pifmaster.ru |
The high cost of industrial and semi-industrial gas generators forces many to make these devices themselves. Moreover, there is nothing particularly complicated about it.
We have prepared links to thematic forums where the manufacture of various models of gas generators is discussed.
There you will also find tips to make it easier to find materials for making this device, as well as various recommendations that will help you choose one or another model for self-production.
Here are links to thematic forums:
- Forumhouse – manufacturing of an automobile gas generator.
- Moonshiners forum - making a gas generator from a pyrolysis boiler.
- Vasdom - making a gas generator and discussing various models.
- OstmetallInfo - manufacturing a gas generator for a forge and discussing its advantages and disadvantages.
Methods of using pyrolysis gas
This gas is used for various needs.
connect a kitchen stove to the gas generator and the fire on it will be only slightly less than that obtained by burning propane or butane.
You can connect an autogen to it (better cleaning will be required) and when oxygen is supplied, the flame temperature will reach two thousand degrees.
electric generators and car engines work well on this gas .
A gasoline car engine, after a little modification, works well on gas produced by a gas generator unit.
A diesel engine can also run on such gas, but a more serious modification of the fuel system will be required.
full power from such an engine , but it will produce ¼ to ½ if the gas generator’s performance is sufficient.
In the context of constantly rising fuel prices, a gas generator installed in the trunk of a car will significantly reduce fuel costs. This is especially true for tractors whose engines operate in the same mode.
However, most often pyrolysis gas is used for heating . After all, 3–4 kilograms of sawdust provide the same thermal energy as 1 m3 of natural gas.
If you can for free or cheap , the savings are quite significant. Therefore, wood processing enterprises, workshops and sawmills can be heated without spending money on purchasing energy resources, because shavings, chips and sawdust appear at such enterprises constantly and in huge volumes.
Due to the high nitrogen content, the calorific value of pyrolysis gas is lower than that of any natural or liquefied gas.
For proper combustion and release of the required amount of heat, it is necessary to increase the gas supply .
To do this, nozzles are drilled out in kitchen stoves and heating boilers without electronic control.
In electronically controlled boilers, the diameter of the jets is increased and the firmware (software) is changed.
In cars, it is necessary to completely redo the fuel system, so the easiest way is to convert carburetor cars to pyrolysis gas.
We recommend converting only inexpensive equipment that is not under warranty.
The use of fuel that does not meet certain standards is often grounds for refusal of warranty repairs .
In addition, it is necessary to convert any equipment to natural gas only after carefully studying forums where users share their experience of such work.
We also recommend that you take fuel cleaning as seriously as possible; this will not only increase its calorific value, but also reduce the risk of clogging the fuel system of the heating device.
on this topic
About the pyrolysis of wood processing waste, watch this video:
Conclusion
Pyrolysis gas, which is produced from sawdust and other wood waste, is an effective and inexpensive fuel .
Therefore, the use of pyrolysis gas generators is justified in cases where there is access to very cheap or free wood waste.
Before describing the process of wood pyrolysis, it is worth giving a general concept of pyrolysis as a process. So, pyrolysis is a chemical reaction of the destruction of a substance caused by exposure to high temperature. Under natural conditions, it occurs together with combustion.
We will show the sequence of the process using wood as an example:
- heating a substance from an external heat source;
- at a temperature of about 300 ° C, the process of decomposition of the substance and the release of flammable hydrocarbons begins;
- since the access of oxygen is not limited, and heat is supplied in the form of an open flame, when reaching 500 ° C the amount of gases increases and they ignite;
- The combustion reaction proceeds independently, without an external heat source. Burnt hydrocarbons provide the required amount of heat for further thermal decomposition of wood.
Scope of application of wood pyrolysis
Ideally, wood pyrolysis occurs in a closed space without oxygen and with a constant supply of heat from the outside. In order not to consume expensive energy resources for this purpose, part of the final product is used to support the process - a mixture of flammable gases. The mixture contains methane, carbon monoxide (CO) and hydrogen; non-flammable substances include carbon dioxide and nitrogen.
Obtaining gaseous fuel from various wood processing wastes is the main area of application of wood pyrolysis in industry.
Example of a wood pyrolysis plant
The main equipment for the technological process is pyrolysis furnaces (gas generators), cooler and filter units. Raw materials in the form of sawdust, wood chips and other waste are loaded into the furnace and burned there with a minimum air supply. Since the performance of the installation directly depends on temperature, industry often uses so-called fast pyrolysis, when the raw material is heated at high speed. The mixture of gases is cooled and filtered, after which it is pumped into tanks for further processing.
Application of pyrolysis in boilers
Pyrolysis boilers are a group of solid fuel units. It differs from traditional direct combustion boilers by having two chambers instead of one. As planned, in the primary combustion chamber, the process of gasification of solid fuel occurs when an insufficient amount of oxygen is supplied, and in the second, the afterburning of the released pyrolysis gases occurs when secondary air is added. But is this how the combustion process actually works? To understand this, we need to consider the design of the heat generator.
At the moment, there are 2 types of pyrolysis boilers; let’s look at the design of each in more detail. The most popular design is when the primary firebox is located above the secondary one. Between them there is a rectangular nozzle made of refractory brick. And now attention: air is pumped into the main firebox using a fan, partially entering the lower chamber for afterburning gases. That is, the principle of pyrolysis is violated from the very beginning, since instead of limiting oxygen, the fan creates an excess of it.
What does this give? Complete and efficient burning of wood, leaving no ash behind. But there is an explanation for this: dry wood does not leave behind ash, but only light ash, half of which is simply blown by a fan through a nozzle into the chimney. Based on all the features of this design, the name “top-blowing boiler” can be assigned, since the fan forces air into the upper chamber. Due to this, the combustion temperature increases, the gas output increases, but it immediately burns out as it passes through the nozzle. This operating algorithm has little in common with the chemical reaction of pyrolysis.
Boilers with natural air supply
In another type of heat generator, the chambers are located in reverse: the main firebox is below, the secondary firebox is above it. There is no nozzle; instead, there is a regular gas duct connecting the chambers to each other. There is no fan here; air is supplied to both fireboxes naturally - due to the draft of the chimney. Moreover, the supply is carried out through separate channels. It should be noted that in this case the process of wood pyrolysis is better organized, combustion in the firebox occurs with low air consumption, its supply is limited by a damper.
About our boilers
Our boilers belong to the second type - they operate on natural draft, burning fuel through the oxidation of exhaust gases using injectors in the combustion chamber.
Wood pyrolysis process
Wood pyrolysis is a process that ensures the decomposition of wood into two components. One of them is the dry residue, which is called charcoal. The second is pyrolysis gas.
It is necessary in order to ensure the functioning of devices such as long-burning boilers.
Such boilers are popular and do an excellent job of maintaining the optimal temperature level even in the coldest winter.
Types of pyrolysis boilers
Wood pyrolysis is a process that is only possible under certain conditions. The first requirement is that there should be no access to oxygen in the chamber in which this action is carried out. The second requirement is that the entire process occurs at sufficiently high temperatures.
Deciduous wood is used as a raw material to produce pyrolysis gas.
Their properties are the most optimal for carrying out this action. However, coniferous species are no exception, although their efficiency is an order of magnitude lower.
The structure in which this action is carried out is a retort. It has a closed shape, and its oxygen level is extremely low. It is equipped with a pipe through which the formed gas is removed. Subsequently, the pyrolysis gas can be burned and ensures the operation of a long-burning boiler.
- 1 Process stages
- 2 Pyrolysis products
Process stages
Pyrolysis fuel is formed as a result of a series of processes, the sequence of which cannot be disrupted. If you neglect at least one of them, then all the work done will not bring any results. It is worth highlighting the main stages of this process:
- Drying. At this stage, the wood is completely stripped of all its moisture content. This action is carried out at temperatures from 0 to 150ºC.
- The pyrolysis process itself. It is at this stage that gas is released and production waste is generated. This stage varies in temperature from 150 to 350ºС. At a temperature of 280ºС, the most effective stage is observed, at which more than half of all raw materials are formed.
- Calcination. At this stage, the separation of resins from charcoal occurs more clearly. In addition, certain gases are released that were previously not subject to temperature parameters. This stage occurs in two stages, and the temperature is 350-550ºС.
Wood itself is a fairly complex component that contains a huge amount of different substances. The pyrolysis process makes it possible to separate them and obtain each in its pure form.
Each temperature stage is characterized by the release of a specific substance. That is why, in order to obtain absolutely all components in their pure form, it is necessary to carry out the pyrolysis process from beginning to end.